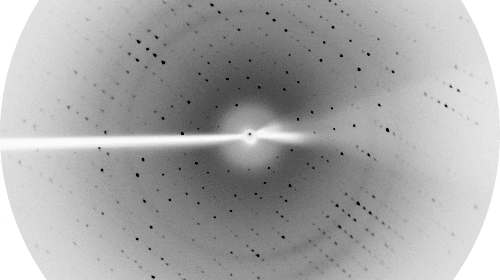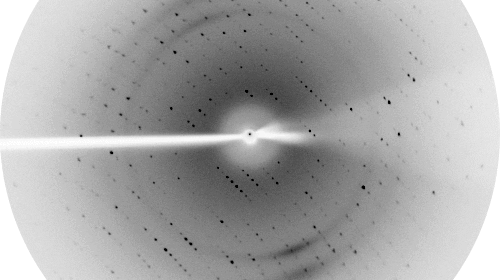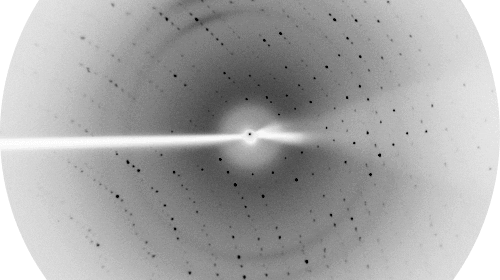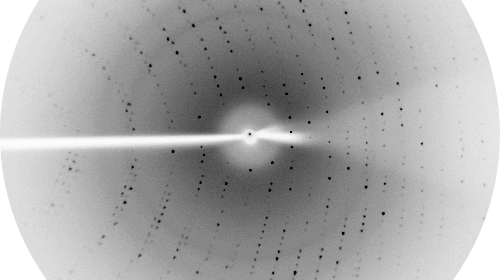
Proteins are the fundamental molecular machines in any living system. Understanding how these machines work requires a knowledge of the three dimensional atomic structure of the protein. The focus of our Laboratory is to understand the molecular and cellular function of various protein systems. Our key experimental technique is X-ray crystallography, although we also utilise a battery of other biochemical and biophysical techniques to enhance our understanding.
The Laboratory has several themes including: metamorphic proteins that undergo dramatic structural changes, light harvesting proteins, molecular machines and spatiotemporal patterning by protein systems. The Laboratory uses the tools of modern molecular biology to prepare proteins of choice. These are then characterised via biophysical and biochemical means prior to structure determination. We use computational and bioinformatic tools to analyse our structures. We are also exploring the physical basis for the structures observed in proteins, structural patterns, their structural transitions and mechanisms of action.